Endoscopic Bariatric and Metabolic Therapies: New and Emerging Technologies
Get the latest insights into emerging endoscopic bariatric and metabolic therapies, including weight loss data from landmark trials.

Inventor of ESG Stomach Tightening
This post provides an updated summary of the 2017 academic paper “Endoscopic Bariatric and Metabolic Therapies: New and Emerging Technologies” by one of our cofounders, Dr. Christopher C. Thompson along with our Scientific Advisory Board member Steven A. Edmundowicz.
Endoscopic bariatric and metabolic therapies (EBMT) represent a new approach to treating obesity. These therapies involve devices and procedures that utilize flexible endoscopy to help patients lose weight or manage blood sugar (for those with pre-diabetes to diabetes). EBMTs can be categorized into stomach and small bowel therapies. This review explores the various EBMTs that are approved in the United States or are undergoing approval by the FDA, as well as their mechanisms of action and their safety and efficacy profiles.
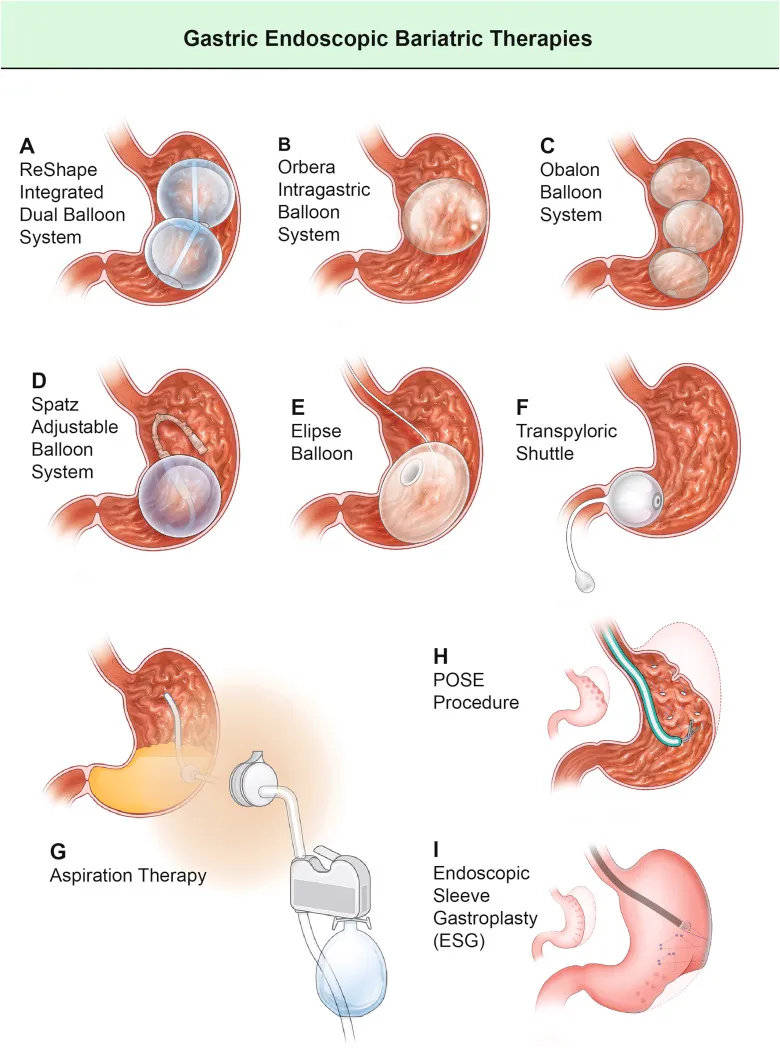
Gastric Endoscopic Bariatric and Metabolic Therapies
Gastric EBMTs are devices and/or procedures performed within the patient’s stomach to help them lose weight.
Intragastric Balloons
Intragastric balloons (IGBs) are devices inserted into the stomach to occupy space, helping patients feel full sooner. Research indicates that an IGB volume of at least 400 mL is necessary for effective weight loss. Some IGBs also slow down the emptying of food from the stomach to the small bowel. Studies show that IGBs can lead to significant weight loss and maintain it after removal.
-
Weight loss: Ranging between 7% to 15% from baseline weight at 6-8 months in clinical trials.
-
Serious adverse events: Abdominal pain, nausea, and vomiting, most of which resolved within a week.
Another intragastric balloon currently under investigation is the Elipse Balloon. The Elipse Balloon, a non-invasive option, is swallowed and naturally deflates after four months, passing through the body. Initial studies showed an average weight loss of 10% total body weight in four months, making it a promising alternative.
Transpyloric Shuttle (TPS)
The TPS system consists of a large and small bulb connected by a flexible tube. The system is deployed through the mouth and located between the stomach and duodenum (the first part of the small bowel). It intermittently causes an obstruction so that food stays in the stomach longer before passing into the small bowel. In a clinical trial, patients who underwent TPS placement lost approximately 10% of their starting weight at 12 months. The device is approved by the FDA but is not commercially available at this time.
Aspiration Therapy
Aspiration therapy involves a device (AspireAssist) that removes a portion of stomach contents after meals, reducing calorie absorption. This method also promotes behavioral changes leading to better eating habits and reduced food intake. The device is approved by the FDA but is not commercially available at this time.
Key Statistics:
-
Weight loss: 14.2% from baseline weight at 12 months in clinical trials.
-
Serious adverse events: Low, including stomach ulcers and skin infection.
Plications and Suturing
These procedures, including primary surgery obesity endoluminal (POSE) and endoscopic sleeve gastroplasty (ESG), reduce the stomach volume by approximately 70% to reduce food intake. POSE utilizes a plication device such as USGI Medical’s POSE2.0, while ESG utilizes a suturing device such as Apollo ESG.
Key Statistics:
-
Weight loss: Ranging between 13% to 14% from baseline weight at 12 months in clinical trials.
-
Serious adverse events: Low, with some incidences of post-procedure pain, nausea, vomiting, and leaks.
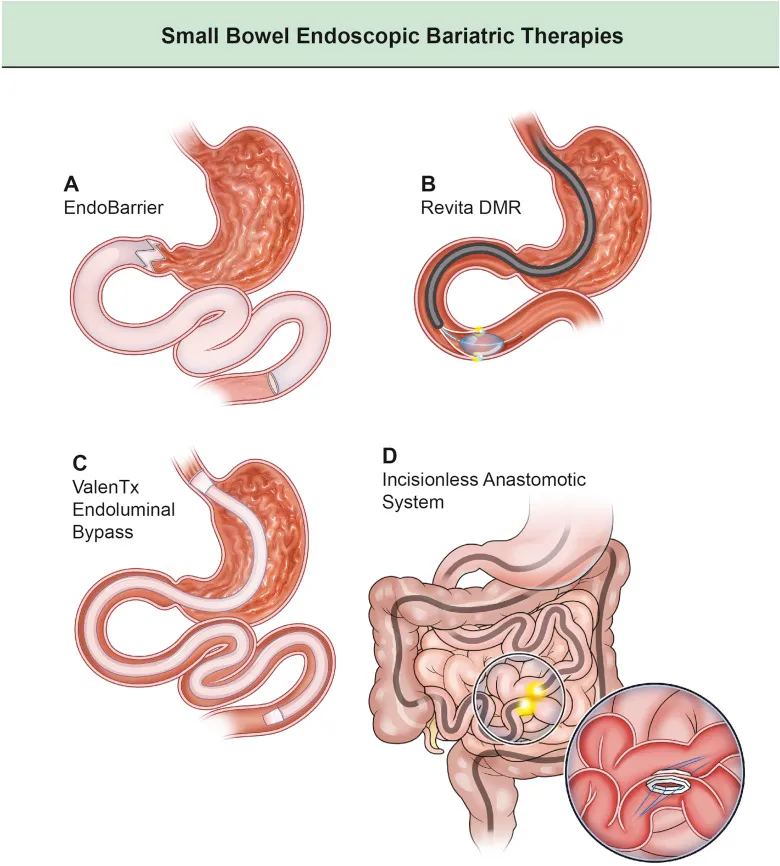
Small Bowel Endoscopic Bariatric and Metabolic Therapies
Small bowel EBMTs focus on altering the small intestine to improve glucose control and/or promote weight loss.
Duodenal-Jejunal Bypass Liner (DJBL)
The DJBL (Endobarrier) is a flexible sleeve inserted through the mouth into the small bowel to prevent food from being fully absorbed, mimicking the effects of gastric bypass surgery, improving glucose control and promoting weight loss. This device is currently not available in the US.
Key Statistics:
-
Weight loss: 19% from baseline weight at 12 months in non-US studies.
-
Serious adverse events: Low, with some incidences of nausea, vomiting, stomach bleeding, and liver abscesses.
Duodenal Mucosal Resurfacing (DMR)
DMR uses hot water to ablate and treat the duodenal mucosa, which has been shown to improve glycemic control without significant weight loss. This device is currently not available in the US.
Key Statistics:
-
Reduction in HbA1c: 1.2% from baseline HbA1c at 6 months.
-
Weight loss: Minimal, around 3% from baseline weight at 6 months.
-
Serious adverse events: Low, including small bowel perforation.
Conclusions
EBMTs offer promising new options for obesity treatment with effective weight loss data and favorable safety profiles. These therapies should be part of a comprehensive weight management program that includes lifestyle modification. As research progresses and more devices receive FDA approvals and become available, EBMTs will become an increasingly important tool in combating obesity.